
PFAS
Content:
PFAS, Per- and polyfluoroalkyl substances, is a large and complex group of over 10000 identified substances with varying properties that are widely used in society. One thing that all PFAS have in common is that they are very difficult to break down. Some PFAS are harmful to both the environment and human health. All PFAS are man-made synthetics that do not occur naturally in the environment.
PFAS have a wide range of applications, including waterproof and dirt-repellent fabrics. PFAS occur everywhere in the environment and are present in the bodies of almost every human being. In Sweden, the population in general is exposed to low levels of PFAS through food and indoor environments. However, local exposure to significantly higher levels occurs in areas where drinking water has been contaminated with PFAS, for example by firefighting foam at fire drill sites. In Europe, some 20 million people have been exposed to drinking water with PFAS levels above the new limits being discussed within the European Union.
PFAS have a wide range of applications, including waterproof and dirt-repellent fabrics. PFAS occur everywhere in the environment and are present in the bodies of almost every human being. In Sweden, the population in general is exposed to low levels of PFAS through food and indoor environments. However, local exposure to significantly higher levels occurs in areas where drinking water has been contaminated with PFAS, for example by firefighting foam at fire drill sites. In Europe, some 20 million people have been exposed to drinking water with PFAS levels above the new limits being discussed within the European Union.
The difficulty involved in breaking down PFAS, combined with the fact that many of the substances are water-soluble and mobile in soil, means that there is a risk that drinking water supplies will continue to be contaminated for many years to come. The dispersal of and exposure to PFAS takes place throughout the substance’s lifecycle, from manufacture to waste management.
Areas of use
PFAS have been manufactured since the 1950s and are used in a wide range of goods and chemical products for their desirable technical properties. Many PFAS repel fat, dirt and water, making them ideal for use in impregnating various textiles, leather and paper packaging. Many are also surfactants, making them useful in the manufacture of products such as detergents, paints, ski wax and cosmetics. Certain PFAS are used in firefighting foam for extinguishing fires involving flammable liquids. These are useful for their ability to quickly form a thin film between the foam and the burning liquid. Other less known applications for PFAS include dental repair materials, medical technical equipment and dirt-repelling agents used in building materials, smartphones and solar cells.
PFAS are resistant to degradation by external factors such as ultraviolet light, other chemical substances or high temperatures. As these are highly effective substances, it often requires only low concentrations to achieve the desired effect in a product.
Area of use | Example uses | Example substances | Example functions |
|---|---|---|---|
Textiles and leather | Outerwear, umbrellas, bags, tents, vehicle upholstery, shoes, carpets, furniture fabrics, impregnation agents | PTFE, FTOH, FTS, PFOA | Water, fat and dirt repellents |
Firefighting foam | Class B foam used on flammable liquids, especially petroleum fires | Fluorinated surfactants, often contain precursors to 6:2 FTS och PFHxA PFOA | Ability to create a thin film between the foam and burning liquid |
Paper and food packaging | Popcorn bags, pizza boxes, cartons, masking paper | PAPs/diPAPs, 6:2 fluorotelomers, PFOA | Fat and water repellent |
Cosmetics | Makeup, sunscreen, moisturiser, foundation cream | PAPs/diPAPs, PFCA | Oil and water repellent, smoothing |
Household goods | Cleaning products, floor polish, car-care products, paint, non-stick pans, ski wax | FTOH, PTFE (Teflon), PFOA, PFHxA | Covers smoothly, non-stick, provides glide and is dirt repellent |
Learn more about the occurrence, function and use of per- and polyfluoroalkyl substances in Swedish Chemicals Agency Report 6/15 (only in Swedish).
What are PFAS?
PFAS, Per- and polyfluoroalkyl substances, is a group of persistent organic substances that all consist of a carbon chain in which hydrogen atoms are entirely or partly replaced by fluorine atoms. Perfluoroalkyl substances have a fully fluorinated carbon chain, while polyfluoroalkyl substances have a partially fluorinated carbon chain.
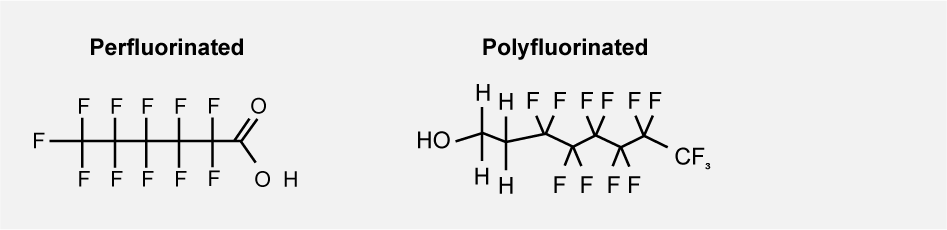
A fully fluorinated carbon chain is called perfluorinated and a partially fluorinated carbon chain is called polyfluorinated.
There is currently no internationally agreed definition of PFAS, but in 2021 OECD (the international organization for economic cooperation and development) concluded on a PFAS definition which has gained wide acceptance. PFAS is here defined as substances that contain at least one fully fluorinated methyl group (-CF3) or fully fluorinated methylene group (-CF2-) without any hydrogen, chlorine, bromine, or iodine atom attached to it. This PFAS definition is also used by the Swedish Chemicals Agency.
Swedish Chemicals Agency Regulations on the obligation to report PFAS to the Products Register (KIFS 2018:4) define PFAS as a molecule that contains one or more fragments consisting of a perfluorinated carbon chain of at least two carbon atoms (C2) with a bond to any atoms or group of atoms. Those substances form a subset of the substances covered by the OECD's PFAS definition. This delimitation has been made for practical reasons and is considered sufficient to obtain relevant information to increase knowledge about how PFAS is used in Sweden.
The term PFAS includes thousands of different substances that can be divided in many groups. The first stage is usually to separate polymers from non-polymers. These two groups can then be divided into several subgroups.
The OECD has thus far identified over 4700 PFAS by CAS number. You can download a table of identified PFAS from the OECD website.
Global Database of PFASs, OECD's website. External link.
External link.
Non-polymeric PFAS
Non-polymeric perfluorinated and polyfluorinated carbon chains are often bound to a functional group. Functional groups are specific groupings of atoms within molecules that have their own characteristic properties, regardless of the other atoms present in a molecule. One example of a functional group is the hydroxyl group consisting of one hydrogen and one oxygen atom (OH). When a hydroxyl group bonds to a carbon chain, an alcohol is formed. Another example is a carboxyl group, a carbon–oxygen double bond and an OH group (COOH), which produces a carboxyl acid.
An example of polyfluorinated carbon chains in a functional group are fluorotelomers, which can be broken down into perfluorinated carbon chains in the environment; for example, 6:2 (the ratio of fluorinated to non-fluorinated carbon atoms) fluorotelomer alcohol (6:2 FTOH) can be broken down into perfluorinated carboxyl acids (PFCAs) in the environment.
Perfluorinated PFAS | Examples |
|---|---|
Perfluorosulfonic acids (PFSA) | PFBS, PFHxS, PFOS |
Perfluorocarboxylic acids (PFCA) | PFBA, PFHxA, PFOA, PFNA, PFDA, PFUnDA |
Perfluoroethers (ethers with less than 20 oxygen bridges) | GenX and Adona |
Branched and/or cyclic perfluorocarbon chains | Branched and/or cyclic perfluorocarbon chains |
Polyfluorinated PFAS | Examples |
|---|---|
Precursors to PFSA och PFCA | 6:2 FTOH, 8:2 FTS |
Polymeric PFAS
PFAS polymers are divided into two subgroups, those with a fluorinated backbone and those with fluorinated side-chains. These fluorinated side-chains can be per- or polyfluorinated.
Polymers | Examples |
|---|---|
Polymers with fluorinated side-chains | FMA |
Fluorpolymers (with a fluorinated backbone) | PTFE (Teflon), PVDF, FED, PFA |
Long-chain and short-chain PFAS
Per- and polyfluoroalkyl substances are sometimes divided into short-chain and long-chain PFAS, based on the length of the fluorinated carbon chain. Among the long-chain PFAS are perfluorooctanoic acid (PFOA) and perfluorooctane sulphonate (PFOS), both of which consist of eight carbon atoms (sometimes referred to as C8 chemistry). Short-chain PFAS include perfluorohexanoic acid (PFHxA) and perfluorohexane sulfonate (PFHxS), both of which consist of six carbon atoms (C6 chemistry).
Although there has been a general perception that short-chain PFAS are less problematic than long-chain forms, this has been proven to be an oversimplified view given that the properties of the molecules are not solely linked to carbon-chain length. Even if stability is primarily due to the extremely strong bond between carbon and fluoride, there are other factors that may affect the stability of the molecule; its structure, for example. Short-chain PFAS ethers are one example of a structural form analogous to long-chain PFAS, as they contain strong oxygen-bridge bonds that join several short perfluorinated chains to form one long, stable chain. Other examples are found in cyclic and branched forms, which remain stable despite not being traditional long-chain PFAS.
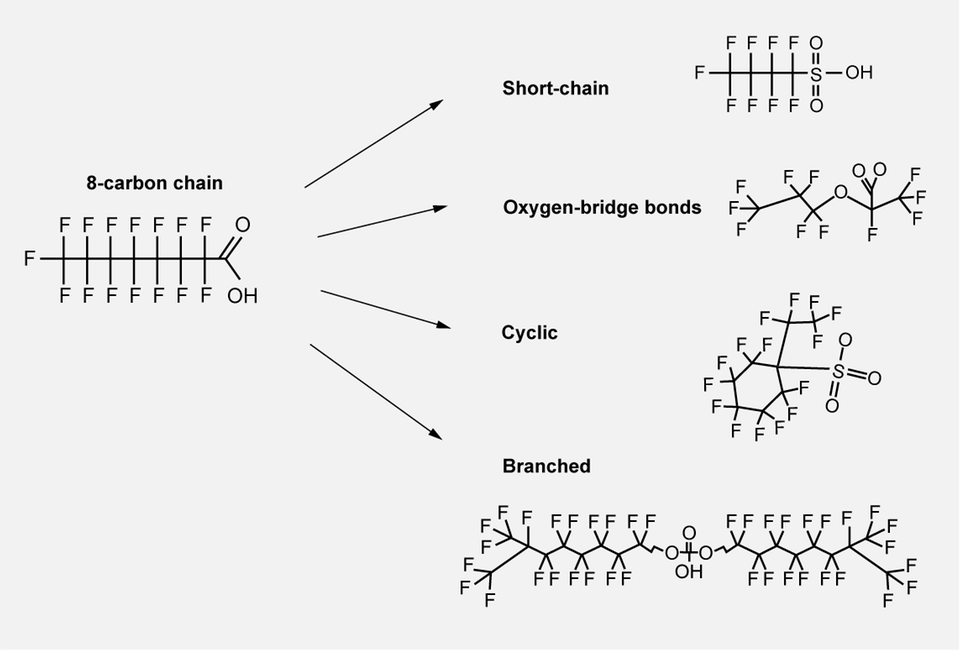
The image shows examples of different structures that are stable despite not being traditional long-chain PFAS.
The properties of PFAS
One thing that all PFAS have in common is that they consist of a carbon chain in which hydrogen atoms are entirely or partly replaced by fluorine atoms. The bond between carbon and fluorine is incredibly strong, making PFAS as a group highly stable, persistent substances. Some PFAS do not degrade at all, while others break down extremely slowly into other PFAS; for example, polyfluorinated side-chains may break down in the environment to form perfluorinated carbon chains such as perfluorooctanoic acid (PFOA) or perfluorohexanoic acid (PFHxA). There have been no studies demonstrating that PFAS degrade completely in the environment, suggesting that these substances will remain in the environment forever.
The substances have a hydrophobic (water repellent) and a hydrophilic (attracted to water) component, making them happy to settle as a layer between, for example, water and an organic solvent or liquid and a solid surface. In certain cases, there is also a group that links the hydrophilic and hydrophobic components. These three components can be varied, meaning that there is enormous potential for developing new PFAS.
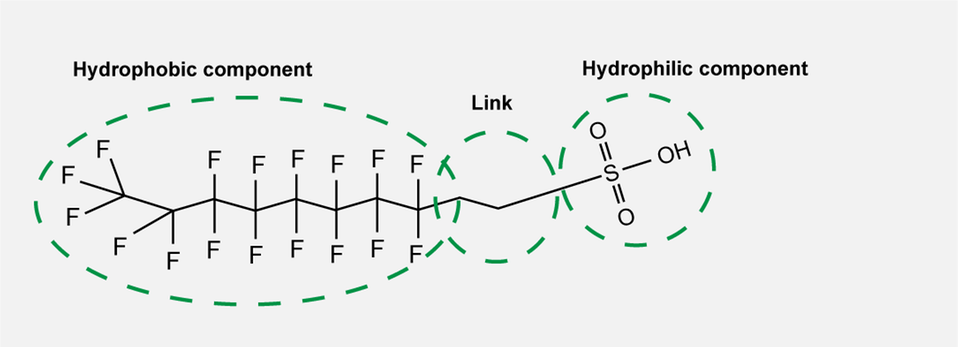
The three different components of PFAS.
Many PFAS are suspected of being harmful and can gradually accumulate in animals and people in a process known as bioaccumulation. Certain PFAS may also be biomagnified; i.e. enriched higher up the food chain. The length of the fluorinated carbon chain can produce different properties that affect the behaviour and characteristics of the substance in the environment and organisms. There is considerable evidence to suggest that the longer the fluorinated carbon chain is, the greater the bioaccumulation.
Unlike many other bioaccumulative substances, fat and water repellent PFAS are not stored in fatty tissue; instead, they bind to proteins and are stored in other bodily organs; for example, the liver and blood. Although there is currently a lack of knowledge regarding how the majority of PFAS affect humans and the environment, there is adequate data to establish that the substances are harmful to health and the environment. This applies to PFOS and PFOA, both of which are classified as suspected carcinogens and reproductive toxins.
Environmental risks
PFAS do not occur naturally in the environment; all PFAS are man-made. All PFAS are extremely persistent in themselves and as degradation products, and therefore accumulate in the environment. PFOA is an example of a persistent, bioaccumulative and toxic substance (PBT).
PFAS may be released into the environment at any time during their lifecycle: from the manufacture of the substance, to the use of the products that contain PFAS, to the point of disposal as waste. As long as we manufacture and use PFAS, these substances will, in some form or other, accumulate over time in the environment.
One thing that all per- and polyfluoroalkyl substances have in common is the ability to disperse over long distances through air and water, meaning that they can be detected far from any areas of manufacture or use – for example, in arctic environments – making PFAS very much a global problem. Different PFAS disperse in the environment in different ways. Volatile variants, such as fluorotelomers, can be dispersed over large areas in the air. Less volatile, ionised forms are largely dispersed in water and bound to particles of organic matter, soil particles for example, or through absorption into living organisms.
Certain PFAS bioaccumulate in living organisms and may then be biomagnified as they rise through the food chain. In fish, a correlation has been shown between the quantity of PFAS the fish accumulate and the length of the carbon chain in the PFAS molecule; long-chain PFAS accumulate but not short-chain. PFAS can also be absorbed by plants but here the inverse applies, with short-chain PFAS accumulating to a greater extent than long-chain.
Health effects
There is evidence that a few PFAS present a health hazard; for example, PFOS and PFOA, which are classified as reproductive toxins and suspected carcinogens. There is however limited knowledge of the health effects of many other PFAS, even if there is good reason to consider all PFAS as a health hazard.
Those studies that have been conducted regarding the health hazards of PFAS are largely experimental animal trials. In studies of mammals, it is common to find effects on the liver, blood lipids. thyroid hormones, the immune system and reproductive system. Other effects observed for individual PFAS include tumours and mammary gland development. Further studies are required in order to establish whether these results are also relevant to humans.
Some observational studies have also been conducted on groups of people who have been exposed to high levels of PFAS as a result of local water contamination. These studies have demonstrated a correlation between increased levels of PFOS and PFOA in the blood and impaired immune function. Researchers have also observed correlations between elevated levels of PFOA in the blood and effects on the liver and cholesterol levels. Effects on birth weight have also been observed.
PFAS in Sweden
The largest identified source of PFAS emissions in Sweden is from the firefighting foam used at fire drill sites. Locally, this practice has given rise to high levels of PFAS in ground, surface and drinking water. Although there are no companies in Sweden manufacturing PFAS, there are those that use the substances in the manufacture of various products in a manner that can cause emissions. Emissions from water treatment plants and waste incinerators and leakage from landfills are other potential sources. Airborne deposition also contributes to increasing levels of PFAS in the Swedish environment.
It has proved to be no easy matter to clean up PFAS contamination in water and soil. With regard to drinking water purification, there are methods available to remove some PFAS; for example, activated carbon filters. Although it is more difficult to remove these substances from soil, research is ongoing.
Legislation and regulation
Individual PFAS are regulated in legislation at global, EU and national level. While there is no comprehensive legislation applicable to all PFAS as a group, there are various regulations covering a handful of specific PFAS. The introduction of regulation relating to a given PFAS often results in the substance in question being replaced by another, unregulated PFAS, meaning that the regulations have no effect in terms of overall risk reduction. This situation, in combination with the extreme persistence and likely hazardous nature of all PFAS, contributes to the overall risk profile. As it is also a time-consuming process to assess each PFAS individually, the Swedish Chemicals Agency and agencies in other EU Member States are working to assess and regulate PFAS as a group. The Swedish Chemicals Agency’s goal is to minimise and eventually end the use of PFAS. Only where there is no alternative and the use is critical to society will PFAS be permitted. In many areas where PFAS are currently in use there are already fluorine-free alternatives available on the market. The Swedish Centre for Chemical Substitution External link. offers guidance to Swedish companies in substituting hazardous chemicals and identifying better alternatives for products and processes.
External link. offers guidance to Swedish companies in substituting hazardous chemicals and identifying better alternatives for products and processes.
CLP Regulation
A few PFAS are covered by harmonised EU classification and labelling requirements. These substances are listed in Annex VI to the Classification, Labelling and Packaging (CLP) Regulation ((EC) No 1272/2008). At present, the following substances are covered: perfluorooctane sulphonate (PFOS), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA) and ammonium perfluorooctanoate (APFO). Work is also currently underway to draft a proposal for the harmonised classification of perfluoroheptanoic acid (PFHpA) and perfluorooctanol 6:2 FTOH. You can search for substances with harmonised classification and labelling in the European Chemicals Agency (ECHA) database C&L Inventory External link..
External link..
REACH Regulation
A dozen or so PFAS are included on the Candidate List of Substances of Very High Concern (SVHC) External link. pursuant to Article 59(10) of the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) Regulation ((EC) No 1907/2006).
External link. pursuant to Article 59(10) of the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) Regulation ((EC) No 1907/2006).
As yet, no PFAS are subject to authorisation requirements and thus included in Annex XIV of the REACH Regulation or the authorisation list on the ECHA website.
From August 2021, Annex XVII of the REACH Regulation contains rules restricting the use of perfluorocarboxylic acids with 9-14 carbon atoms in the chain (C9-C14 PFCAs), their salts and C9-C14 PFCA-related substances. The restriction covers around 200 PFAS and the new rules will apply from 25 February 2023, with certain exceptions.
The Public Activities Coordination Tool (PACT) External link. provides an overview of substances undergoing some form of evaluation under the REACH or CLP Regulations. This list, which is compiled by the ECHA, contains a number of PFAS. Work is currently underway to draw up restrictions for perfluorohexanoic acid (PFHxA) and perfluorohexane sulfonate (PFHxS). Work is also in progress to ban PFAS in fire-fighting foams.
External link. provides an overview of substances undergoing some form of evaluation under the REACH or CLP Regulations. This list, which is compiled by the ECHA, contains a number of PFAS. Work is currently underway to draw up restrictions for perfluorohexanoic acid (PFHxA) and perfluorohexane sulfonate (PFHxS). Work is also in progress to ban PFAS in fire-fighting foams.
There is consensus within the EU that PFAS should be treated as a single group. Sweden has therefore joined a number of other Member States in starting work to develop a broad restriction proposal in Annex XVII to the REACH Regulation, covering all PFAS in addition to those already subject to regulation.
All stakeholders have the opportunity to offer viewpoints that can be used as a basis for developing new restrictions or other regulations. You can comment on proposed amendments to the REACH Regulation here. External link.
External link.
Stockholm Convention and POPs Regulation
The Stockholm Convention on Persistent Organic Pollutants (POPs) lists over a hundred substances that can degrade to perfluorooctane sulphonate (PFOS), perfluorooctanoic acid (PFOA) and PFOA salts and approximately 800 substances that can degrade to (PFOA). The Stockholm Convention's requirements for the restriction of PFOS and PFOA and substances that can degrade to these substances have been incorporated into EU legislation through the so-called POPs Regulation ((EU) 2019/1021).
You can learn more about the POPs Regulation here External link..
External link..
It is expected that perfluorohexane sulfonate (PFHxS), its salts and related substances will be included in the Stockholm Convention during 2022, resulting the phasing out of these substances globally.
| Substance/substancegroup | CAS-number | REACH: Annex XVII | REACH: Candidate List | CLP:Annex VI | POPs |
|---|---|---|---|---|---|
| Perfluorooctanoic acid (PFOA), its salts and PFOA-related compounds | 335-67-1, and others | X(only335-67-1) | X | X | |
| Perfluorooctane sulfonic acid (PFOS), and its derivatives | 1763-23-1, and others | X | X | ||
| Ammonium pentadecafluorooctanoate (APFO) | 3825-26-1 | X | X | ||
| Perfluorohexane-1-sulphonic acid (PFHxS), and its salts | 355-46-4, and others | X | |||
| C9-C14 PFCA-related substances | X(apply from 25 February 2023) | ||||
| Perfluorononan-1-oic-acid (PFNA), and its sodium and ammonium salts(C9 PFCA) | 375-95-1, and others | X | X | X | |
| perfluorononan-1-oic acid (PFDA), and its sodium and ammonium salts(C10 PFCA) | 335-76-2, and others | X | X | X | |
| Henicosafluoroundecanoic acid (PFUnDA)(C11 PFCA) | 2058-94-8 | X | X | ||
| Heptacosafluoro-tetradecanoic acid (PFTeDA)(C14 PFCA) | 376-06-7 | X | X | ||
| Pentacosafluorotridecanoic acid (PFTrDA)(C13 PFCA) | 72629-94-8 | X | X | ||
| Tricosafluoro-dodecanoic acid (PFDoDA)(C12 PFCA) | 307-55-1 | X | X | ||
| HFPO-DA (GenX) | 13252-13-6, and others | X | |||
| Perfluorobutane sulfonic acid (PFBS), and its salts | 375-73-5, and others | X |
Reporting PFAS to the Products Register
In Sweden, there is a statutory requirement to report PFAS that are deliberately added to chemical products to the Swedish Chemicals Agency’s Products Register. This requirement applies to those who manufacture or import notifiable products, irrespective of the percentage of the substance in that product. One aim of reporting is to increase knowledge about which PFAS are being used in chemical products available on the Swedish market.
Phase out the use of PFAS with the PRIO tool
There are alternatives available for many areas where PFAS substances are currently used. Many companies also want to keep ahead of current legislation and as far as possible phase out PFAS from their products.
In the Swedish Chemical Agency’s PRIO-tool there is a database containing almost 11 000 PFAS substances. The database enables manufacturers to identify PFAS ensuring their products are PFAS-free. The list of PFAS substances can also be used as support when setting chemical requirements for buyers and procurers who want to avoid PFAS. Via the link below, you get a complete search result of PFAS substances that you can easily export to an Excel file.
Download PRIO's list of PFAS substances
The list of PFAS substances in PRIO should not be taken as a complete list of all PFAS substances found world-wide, but it provides examples of almost 11,000 PFAS substances that meet the OECD's 2021 PFAS definition and are therefore unique in its kind. It is also the OECD's definition of PFAS that was used in the EU restriction proposal on prohibiting all manufacture and sale of PFAS in the EU that has been submitted to Echa by Sweden and four other member states.
The Swedish Chemicals Agency's work with PFAS
There is still a lack of knowledge about many PFAS substances, but much data indicate that all PFAS can have harmful effects on health and the environment. That's why we at the Swedish Chemicals Agency are working towards minimizing the use of PFAS and eventually phase it out completely. We are also part of several national and international collaborations where we work to increase knowledge and awareness about PFAS.
Proposal to restrict the use of PFAS
In February 2023 the Swedish Chemicals Agency, together with four other European authorities, presented a broad limitation proposal for PFAS in the EU. The basis for the proposal is that the current knowledge about PFAS should be used to assess and regulate all PFAS substances as one group and that PFAS should only be allowed where it is necessary to maintain health and safety or where it is otherwise critical to society and where there are no available alternatives. Right now the European Chemicals Agency, ECHA, is evaluating the proposal. A public consultation on the proposal will be open from March 17, 2023 and six months onward.
A restriction of PFAS is expected to enter into force in 2025 at the earliest.
Read more about the restriction proposal.
Read the restriction proposal at ECHA's website External link.
External link.
Collaborations on PFAS
An important part of the work with PFAS is a broad collaboration with various authorities and other stakeholders, both in Sweden, the Nordic countries, within the EU and globally. This collaboration mainly takes place within the framework of various networks. There is one national authority network and one network with relevant stakeholders in Sweden, such as researchers, water producers and municipalities. We are also part of a network at EU level that the European chemicals agency ECHA manages.
Information held by other government agencies
In Sweden, there are many government agencies involved in supervision and regulatory development in areas where PFAS occur: the Swedish Food Agency is the supervisory authority for drinking water quality, as well as materials that come into contact with foodstuffs; the Swedish Environmental Protection Agency is responsible for environmental monitoring; while the Swedish Civil Contingencies Agency (MSB) is responsible for civil preparedness and also provides professional training for fire and rescue services. Our guide PFAS – Which Authority Does What? (in Swedish) will help you to find the various agencies’ information on PFAS.
There is also useful information about PFAS available from authorities and organisations outside Sweden; for example, the European Chemicals Agency (ECHA) and the Organisation for Economic Co-operation and Development (OECD).
ECHA Perfluoroalkyl chemicals (PFAS). External link.
External link.
OECD Portal on Per and Polyfluorinated Chemicals. External link.
External link.
Advice to stakeholders
The issue of PFAS affects many societal stakeholders. Our guide Finding the Right Information on PFAS (in Swedish) provides advice and information aimed at various stakeholders; for example, drinking water producers, supervisory authorities and those who manufacture and sell products containing PFAS. You will also find information for private individuals such as those with their own well.
More information
New EU restriction can stop all manufacture and sale of PFAS
Read about PFAS i cosmetics in PM 9/21: PFASs in cosmetics. , 1.7 MB.
, 1.7 MB.
